Abstract
The E26 transformation-specific (ETS) transcription factor (TF) family member Friend Leukemia Virus Integration 1 (Fli-1) is linked to the oncogenesis of virally induced leukemia and Ewing's Sarcoma, as well as the development of autoimmune diseases including systemic lupus erythematosus and systemic sclerosis. A recent report presents compelling evidence that Fli-1 restrains CD8 effector T cell differentiation and protective immunity against cancer. How Fli-1 regulates primary T cell differentiation and function remain largely elusive. In this study, we investigated the role of Fli-1 in T-cell pathogenicity of graft-versus-host disease (GVHD) and validated Fli-1 as a therapeutic target.
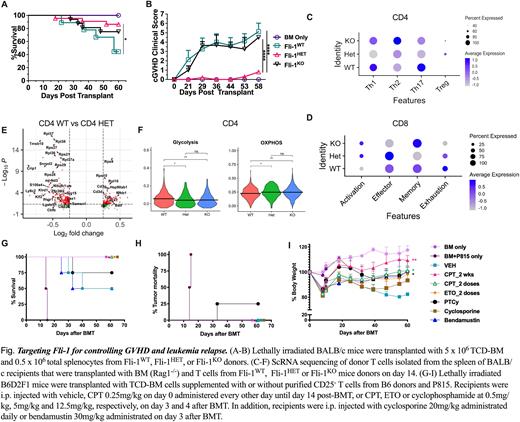
Using conditional knock-out models featured as homozygous (Fli-1KO) or heterozygous (Fli-1HET) deficiency of Fli-1 in T cells, we found that Fli-1 dynamically regulates different T-cell subsets in allogeneic responses and pathogenicity in the development of acute and chronic GVHD. Compared to Fli-1KO or Fli-1WT T cells, Fli-1HET T cells induced the mildest aGVHD and cGVHD supported by lowest Th1 and Th17 differentiation (Fig. 1 A-B). In addition, Fli-1HET T cells were able to maintain a strong graft-versus-leukemia (GVL) effect while inducing significantly attenuated GVHD. Fli-1-deficient CD8+ T cells expressed comparable levels of cytolytic molecules, reduced levels of exhaustion markers, and differentiated more into memory precursor effector cells compared to WT CD8 T cells after been transferred into allogeneic recipients.
To further understand how Fli-1 regulates T-cell gene transcription and heterogeneity, we performed scRNA seq analysis of donor T cells, including Fli-1WT, Fli-1HET and Fli-1KO, isolated from recipient spleens. The scRNA seq analysis on the donor T cells indicated a major difference in the transcriptional regulation by Fli-1 in CD4 versus CD8 T cells and Fli-1 gene dose dependent modification in transcriptional pathways in Fli-1Het vs Fli-1KOT cells. Fli-1 deemed to play a distinct role in regulating gene transcription in CD4 versus CD8 T cells, in that more activation and less exhaustion gene pathways were enriched in CD8 Fli-1 deficient cells, while fewer genes in Th1/Th17 pathogenic pathways were enriched in CD4 Fli-1 deficient cells (Fig. 1 C-D). Overall, Fli-1 deficiency increased gene enrichment in OXPHOS pathway in both CD4 and CD8 T cells, and reduced gene enrichment in glycolysis pathway majorly in CD4 T cells (Fig. 1 E-F). Consistent with the lowest pathogenicity of Fli-1Het T cells for GVHD induction, Fli-1Het CD4 T cells had the lowest Th1/Th17 pathway gene enrichment and TCR pathway downstream gene expression.
Camptothecin, topotecan or etoposide exhibited action as potent Fli-1 inhibitors at low-doses. For translational purpose, we tested these drugs and found that each of these drugs significantly attenuated GVHD severity while preserving the GVL effect. The efficacy of camptothecin or etoposide at low doses was dependent of Fli-1 expression on donor T cells. Mechanistically, blocking Fli-1 suppressed activated effector T cells while sparing Tregs and had no side effect on hematopoietic stem cells. Importantly, using the same treatment schedule as post-transplantation cyclophosphamide (PTCy), two doses of camptothecin or etoposide administered on day 3 and 4 were sufficient for the prevention of GVHD and leukemia relapse, in contrast to GVHD mortality in some of those treated with bendamustine and leukemia relapse in some of those treated with PTCy or cyclosporine (Fig. 1 G-I). In addition, the beneficial effect of camptothecin was extended in GVHD induced by human T cells in a xenograft model.
In conclusion, we provide the first evidence that Fli-1 plays a crucial role in alloreactive CD4 T-cell activation and differentiation, and that targeting Fli-1 may be an attractive strategy for treating GVHD without compromising GVL effect.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.